Box Turtle Bulletin
 News and commentary about the anti-gay lobby
News and commentary about the anti-gay lobby News and commentary about the anti-gay lobby
News and commentary about the anti-gay lobbyJune 23rd, 2010
HIV is a very deceptive virus. One way in which it is so insidious is that once it enters a human body, it can take the body several weeks to develop enough antibodies for the infection to show up on most existing HIV tests today. That also just happens to coincide with one of the periods in the virus’ life cycle in which the newly infected person is most infectious and capable of passing it on to others through unsafe sex. Once the body produces enough anti-bodies to show up on a typical HIV test, it also has, ironically, begun to fight off the virus enough so that the individual is somewhat less capable of passing it on to others — although even then that risk is still very far from zero. What that means is that between the time of initial infection and the buildup of antibodies, that person is at one of his most infectious stages in the disease and if he is tested, his test result would likely still be negative during that early period.
There are tests known as nucleic acid testing (NAT) which can detect the presence of the virus itself, but they aren’t routinely used because of their high false-positive rate. But a study published in the June 15 issue of the Annals of Internal Medicine found that adding NAT testing to the current antibody-based rapid HIV testing that is commonly in use can increase detection of HIV by 23%. Yesterday, the Food and Drug Administration announced the approval of the first HIV test to detect both the antibodies and the HIV virus itself. According to a statement from the FDA:
“The approval of this assay represents an advancement in our ability to better diagnose HIV infection in diagnostic settings where nucleic acid testing to detect the virus itself is not routinely used,” said Karen Midthun, M.D., acting director of FDA’s Center for Biologics Evaluation and Research. “It provides for more sensitive detection of recent HIV infections compared with antibody tests alone.”
The test was developed by Abbott Laboratories. The separate study published in the Annals of Internal Medicine, which appears to be unrelated to the FDA announcement, was funded by the National Institutes of Health, University of California at San Diego, and the California HIV/AIDS Research Program.
Latest Posts
Featured Reports
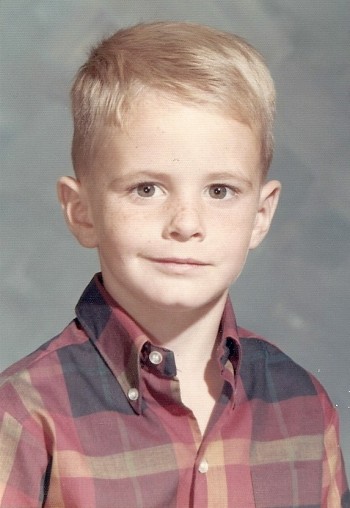 In this original BTB Investigation, we unveil the tragic story of Kirk Murphy, a four-year-old boy who was treated for “cross-gender disturbance” in 1970 by a young grad student by the name of George Rekers. This story is a stark reminder that there are severe and damaging consequences when therapists try to ensure that boys will be boys.
In this original BTB Investigation, we unveil the tragic story of Kirk Murphy, a four-year-old boy who was treated for “cross-gender disturbance” in 1970 by a young grad student by the name of George Rekers. This story is a stark reminder that there are severe and damaging consequences when therapists try to ensure that boys will be boys.
When we first reported on three American anti-gay activists traveling to Kampala for a three-day conference, we had no idea that it would be the first report of a long string of events leading to a proposal to institute the death penalty for LGBT people. But that is exactly what happened. In this report, we review our collection of more than 500 posts to tell the story of one nation’s embrace of hatred toward gay people. This report will be updated continuously as events continue to unfold. Check here for the latest updates.
In 2005, the Southern Poverty Law Center wrote that “[Paul] Cameron’s ‘science’ echoes Nazi Germany.” What the SPLC didn”t know was Cameron doesn’t just “echo” Nazi Germany. He quoted extensively from one of the Final Solution’s architects. This puts his fascination with quarantines, mandatory tattoos, and extermination being a “plausible idea” in a whole new and deeply disturbing light.
On February 10, I attended an all-day “Love Won Out” ex-gay conference in Phoenix, put on by Focus on the Family and Exodus International. In this series of reports, I talk about what I learned there: the people who go to these conferences, the things that they hear, and what this all means for them, their families and for the rest of us.
Prologue: Why I Went To “Love Won Out”
Part 1: What’s Love Got To Do With It?
Part 2: Parents Struggle With “No Exceptions”
Part 3: A Whole New Dialect
Part 4: It Depends On How The Meaning of the Word "Change" Changes
Part 5: A Candid Explanation For "Change"
 At last, the truth can now be told.
At last, the truth can now be told.
Using the same research methods employed by most anti-gay political pressure groups, we examine the statistics and the case studies that dispel many of the myths about heterosexuality. Download your copy today!
And don‘t miss our companion report, How To Write An Anti-Gay Tract In Fifteen Easy Steps.
 Anti-gay activists often charge that gay men and women pose a threat to children. In this report, we explore the supposed connection between homosexuality and child sexual abuse, the conclusions reached by the most knowledgeable professionals in the field, and how anti-gay activists continue to ignore their findings. This has tremendous consequences, not just for gay men and women, but more importantly for the safety of all our children.
Anti-gay activists often charge that gay men and women pose a threat to children. In this report, we explore the supposed connection between homosexuality and child sexual abuse, the conclusions reached by the most knowledgeable professionals in the field, and how anti-gay activists continue to ignore their findings. This has tremendous consequences, not just for gay men and women, but more importantly for the safety of all our children.
Anti-gay activists often cite the “Dutch Study” to claim that gay unions last only about 1½ years and that the these men have an average of eight additional partners per year outside of their steady relationship. In this report, we will take you step by step into the study to see whether the claims are true.
Tony Perkins’ Family Research Council submitted an Amicus Brief to the Maryland Court of Appeals as that court prepared to consider the issue of gay marriage. We examine just one small section of that brief to reveal the junk science and fraudulent claims of the Family “Research” Council.
 The FBI’s annual Hate Crime Statistics aren’t as complete as they ought to be, and their report for 2004 was no exception. In fact, their most recent report has quite a few glaring holes. Holes big enough for Daniel Fetty to fall through.
The FBI’s annual Hate Crime Statistics aren’t as complete as they ought to be, and their report for 2004 was no exception. In fact, their most recent report has quite a few glaring holes. Holes big enough for Daniel Fetty to fall through.
Daniel Brookshire
June 24th, 2010
I applaud this new test procedure, but it worries me that it has a history of a lot of false positives! That would really be unnerving if someone went in and showed up positive when they really weren’t. Is there further info on how the test has been improved so as to give reliable results?
Leave A Comment